Introduction
Ischaemic heart disease, a major public health issue, is responsible for one in every six deaths and an estimated 1,065,000 will have an acute coronary syndrome (ACS) annually.1 ACS is an umbrella term that comprises unstable angina, non-ST segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI).2
NSTEMI is the commonest form of acute myocardial infarction and a leading global cause of premature morbidity and mortality.3 Recent studies have demonstrated a significant reduction in the incidence of STEMI over recent years whereas NSTEMI incidence has remained stable.3 Annual mortality rates from Europe have shown a non- significant mortality difference between STEMI and NSTEMI being 9 % and 11.6 % respectively.4 This has been echoed in Australian literature with the recent Acute Coronary Syndrome Prospective Audit (ACACIA) registry showing one-year mortality of 8 % for STEMI and 10.5 % for NSTEMI.5
Role of PCI
Following Gruentzig’s first angioplasty in 1977 the growth of percutaneous intervention (PCI) with the use of stents has been exponential. Figures from Europe show that the number of stenting procedures increased from 3,000 in 1992 to 777,000 in 2004.6 However, this exponential increase has not been met with a corresponding reduction in mortality across all presentations of ischaemic heart disease. Clearly PCI has beneficial effects in specific patient populations but it is not a universal panacea without consequence.
In the STEMI cohort, the evidence for culprit vessel PCI over thrombolysis is well-established with PCI offering a more effective strategy for improving both short- and long-term outcomes.7 However, management of non-culprit disease is not so straightforward. The recent Preventive Angioplasty in Myocardial Infarction (PRAMI)8 and Complete versus Culprit-Lesion only PRimary PCI Trial (CvLPRIT)9 trials have demonstrated improved outcomes for patients with complete revascularisation versus medical therapy, however this remains controversial.
In the NSTEMI cohort, the influence of PCI in determining outcome remains contentious. However, the majority of evidence supports the use of PCI to improve short-term outcomes, particularly the need for repeat revascularisation.10 There is also evidence for a survival advantage offered by PCI in certain high-risk NSTEMI subgroups.11
However, despite clear benefits, PCI is not without complications. Being an invasive procedure, there is an inherent risk involved with PCI that has been quoted as between 1 % and 2 %.12 Notwithstanding this risk, there are further complications that can occur as a direct result of coronary intervention that affect the distal coronary microcirculation. These include distal embolisation, microvascular spasm, microvascular thrombosis, inflammation and ischaemia- reperfusion injury.13 Such complications can lead to myocardial ischaemia and infarction. Some investigators have demonstrated that myocardial infarction as a consequence of PCI is associated with reduced survival.14 Therefore, the decision to stent a coronary stenosis is not to be taken without due consideration.
Revascularisation Strategies in Patients with ACS
Invasive management strategies are generally delivered around visual interpretation of coronary stenoses and revascularisation decisions mediated at the discretion of the angiographer. Previous studies dating back almost 40 years, as well as recent Fractional Flow Reserve (FFR) studies, have challenged this visual approach.15,16 It has been effectively demonstrated that judgements as to the haemodynamic severity of coronary stenoses performed visually are subjective and inaccurate, leading to misdiagnosis and altered treatment decisions, which can be of prognostic significance.17,18
Whilst revascularisation decisions are frequently straightforward, in cases of acute STEMI it can often be difficult to identify the infarct-related artery, especially in cases of UA/NSTEMI when no localising ECG changes have been observed and no regional wall motion abnormalities are notable. Moreover, there are well-founded concerns that the angiographic severity of non-culprit lesions may be overestimated in ACS due to diffuse vasoconstriction poorly responsive to conventional vasodilators.
A study evaluating lesion severity in non-culprit vessels in 48 patients imaged within nine months post-STEMI demonstrated that lesion severity decreases with time (presumably as thrombus is resorbed and vascular tone normalises), with minimal lumen diameter on quantitative coronary angiography (QCA) improving from 1.53 ± 0.51 mm to 1.78 ± 0.65 mm, (P<0.001) and diameter stenosis improving from 49.3 ± 14.5 % to 40.4 ± 16.6 %, (P<0.0001).19 This is of concern for patients with NSTEMI where identifying the culprit lesion can be difficult and thus interpreting the angiogram may be even more challenging.
Multi-Vessel Disease
Multi-vessel coronary disease (MVD) is observed in approximately 30–50 % of patients presenting with acute ST elevation myocardial infarction and is associated with a worse prognosis.20 In the recently published PRAMI trial, 54 % of STEMI cases had MVD as defined by a stenosis of 50 % or more in one or more coronary arteries other than the infarct-related artery.8 Likewise, in patients with non-ST elevation myocardial infarction, 30–59 % of patients have MVD.21 In patients with STEMI and MVD, the culprit artery is generally obvious but the functional significance of non-culprit lesions may be difficult to determine. In patients with NSTEMI and MVD, the same scenario applies but often with the additional difficulty of correctly identifying the culprit itself. The ability to accurately assess the functional significance of non-culprit stenoses at the time of initial angiography would potentially facilitate revascularisation decisions with potential for health and economic benefits.
FFR
Fractional flow reserve is a whole-cycle, pressure-derived index that assesses the haemodynamic importance of an epicardial stenosis and can be performed at the time of coronary angiography, guiding appropriate revascularisation decisions. Indeed, amongst lesions that are less than 90 % in severity, current guidelines mandate the demonstration of ischaemia prior to revascularisation.22 Both the recent AHA and ESC guidelines have therefore given FFR Level A evidence for guiding revascularisation strategy in intermediate lesions.17 With the potential but real complications of unnecessary PCI, being certain of a lesion’s need for revascularisation assumes significant importance.
Validation of FFR
Following initial exploratory work in animal models,17 numerous clinical studies comparing FFR with non-invasive stress tests established an ischaemic FFR threshold of ≤0.75.25,26 Pijls and colleagues examined 41 consecutive patents with an intermediate lesion on coronary angiography and compared FFR with exercise bicycle stress testing, dobutamine stress echocardiography and thallium scintigraphy.25 All patents with an FFR value ≤0.75 demonstrated reversible myocardial ischaemia in at least one non-invasive modality. In contrast, 21 of the 24 patients with an FFR≥0.75 had negative testing for reversible ischaemia on all three stress tests. The sensitivity of FFR at predicting reversible ischaemia was 88 %, specificity 100 % with a positive predictive value of 100 %; the overall accuracy was 93 %.25 Likewise FFR values ≥0.80 are associated with negative ischaemic results with a predictive accuracy of 95 %.27 An important consideration is that the validating studies for FFR were performed in highly selected patients with single-vessel coronary artery disease with normal left ventricular function and no previous myocardial infraction. Thus it becomes difficult to extrapolate the validity of FFR outside of these strict parameters to other patient populations e.g. ACS.
Use of FFR in Patients with ACS: The Ability to Achieve Maximal Hyperaemia
A fundamental aspect of FFR is the ability to achieve maximal hyperaemia in order to achieve a near linear relationship between pressure and flow in the physiological blood pressure range.28 Maximal coronary hyperaemia is dependent on an intact microcirculation and an adequate hyperaemic stimulus.28 Factors affecting the coronary microcirculation (e.g. severe left ventricular hypertrophy) may impact on the ability to achieve maximal hyperaemia.28 Myocardial infarction (MI) can affect the distal coronary microcirculation secondary to a variety of mechanisms that include distal embolic phenomenon, microvascular stunning and acute ischaemic microvascular dysfunction.30 Due to the heterogeneous nature of MI, this effect may vary according to the size of myocardial infarction and the time from infarction to FFR assessment. Thus the validity of utilising FFR in patients with recent MI is not fully established. Positron emission tomography (PET) studies have demonstrated impaired microcirculatory function in the infarcted territory compared with healthy controls up to six months following acute myocardial infarction (AMI).31 Thus in patients with recent myocardial infarction (MI), microvascular injury, stunning and oedema can result in a failure to achieve minimal resistance and FFR values may be falsely elevated.31
The effect of acute microcirculatory impairment is exemplified in acute STEMI, a syndrome characterised by distal embolisation, inflammation and injury to the microcirculation. Tamita and colleagues described a higher post-PCI FFR in acute STEMI patients compared with stable CHD with similar IVUS-derived lesion parameters. Patients with more pronounced microcirculatory dysfunction as demonstrated by a reduction in TIMI flow (TIMI II) also had a higher FFR compared with those patients with TIMI III flow.32 Furthermore by using a surrogate of a hyperaemic response in 40 patients with acute STEMI, it has been shown that such patients have lowered vasodilatory capacity when compared to stable patients. Intuitively, there was also lower coronary flow reserve and higher IMR in acute STEMI compared with stable angina (SA) and NSTEMI.33 Thus in patients with acute STEMI the assessment of FFR in the culprit vessel is not recommended.
In NSTEMI, several small studies have shown that FFR assessment is reliable and valid from between four and six days following the index event34–35 and the larger, multinational Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) study also included patients with NSTEMI.36 However these patients were generally stable and had unreported infarct sizes. Using the resistive reserve ratio (RRR), a surrogate measure of the ability to achieve maximal hyperaemia, our group effectively demonstrated that amongst selected patients with NSTEMI, the vasodilatory capacity in the culprit vessel was not significantly different from that of stable angina [SA 2.8 (1.7–4.8) versus NSTEMI [2.46 (1.6–3.9); p=0.75]. Furthermore the index of microvascular resistance (IMR) was not significantly higher in NSTEMI compared with stable angina, again suggesting that the degree of microvascular dysfunction in NSTEMI was not prohibitive for hyperaemia (NSTEMI 22.7 ± 11.3 vs. SA 18.3 ± 9.2; p=ns).33
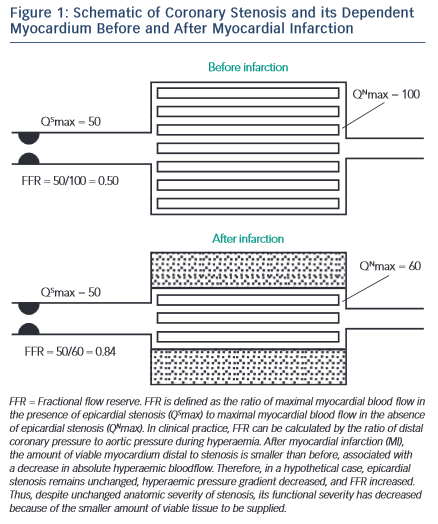
FFR Use in Late AMI (see Figure 1 )
As the microcirculation recovers, so does the ability to achieve maximal hyperaemia, and FFR measurement made at this timepoint reflects a definitive reduction in perfused myocardium rather than a transient phenomenon. De Bruyne et al. highlighted this in 57 patients who were ≥6 days after AMI comparing single-photon emission computed tomography (SPECT) performed before and after PCI with FFR. They established 100 % specificity using an FFR cut-off value of 0.75 against truly positive and truly negative SPECT, i.e. tests that were positive that reverted to completely negative post-PCI. 37 Other groups have shown that rather than recover, the microcirculation remains persistently abnormal in both the culprit and non-culprit territories for up to six months following myocardial infarction. 31 However, these data originate from a small number of patients and predate the later FFR threshold studies and thus, whilst thought-provoking, do not detract from the diagnostic capacity of FFR in chronic MI.
FFR in Non-Culprit Vessels
Ntalianis et al. performed a small prospective cohort analysis on 75 STEMI and 26 NSTEMI patients with non-culprit FFR performed post-PCI to the culprit vessel.38 FFR was repeated at 35 ± 4 days post- procedure. There was no significant difference in percentage stenosis or minimal luminal area between studies despite improvements in left ventricular ejection fraction (LVEF). There was also no demonstrable difference in FFR between studies and in only two lesions did an FFR>0.80 decrease to <0.75. The lack of change in FFR was not affected by the improvement in LVEF. This was an important study as it demonstrated that FFR could be used accurately in non-culprit lesions in patients with ACS when measured acutely, important in considering the design of future studies.
Current FFR Thresholds In ACS
There have been several studies that have aimed to establish and validate FFR thresholds for ischaemia in patients with ACS. In 48 stabilised patients with recent MI, Samady and colleagues compared FFR in the infarct-related artery to non-invasive findings using SPECT and myocardial contrast echocardiography (MCE).34 Patients had a mean time to angiography of 3.7 days with 73 % of patients with STEMI.
The group demonstrated that an FFR ≤ 0.75 had 91 % sensitivity, 93 % specificity and a diagnostic accuracy of 92 % for detecting reversible ischaemia. They provided an optimal cut off FFR value of ≤0.78 for detecting reversible ischaemia using receiver-operating characteristic (ROC) analysis. De Bruyne et al. demonstrated that an FFR ≤0.75 in a culprit vessel ≥6 days following an AMI was still predictive of reversible ischaemia shown on non-invasive SPECT imaging.31 More recently, and in the largest NSTEMI cohort studied to date, we have demonstrated that the current thresholds for ischaemia remain valid for NSTEMI.39
Specifically, FFR values were compared with stress perfusion MRI at an average of four days following the acute infarct. The overall sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for FFR ≤ 0.8 were 91.4 %, 92.2 %, 76 % and 97 % respectively. Diagnostic accuracy was 92 %. The optimal cut- off value for FFR to identify inducible perfusion defects on MRI was <0.805 and this had high diagnostic accuracy (AUC 0.94, p<0.001). Thus, there is emerging evidence for the use of FFR to determine ischaemia in the culprit territory four to six days following ACS. Clearly although the resistance indices can be higher in NSTEMI compared with stable patients, it appears that the microcirculation can dilate sufficiently to enable maximal hyperaemia and allow valid FFR measurements.
FFR in NSTEMI
The potential clinical utility of using FFR to guide decision making in NSTEMI was investigated by Carrick and colleagues.17 They performed a retrospective study of 100 patients where FFR was used in the clinical case. The angiograms were analysed by five cardiologists who made an initial treatment decision. Following FFR disclosure the cardiologists were asked to re-evaluate their original decision. The use of FFR in this manner led to an increase in prescription of medical therapy and improved conformity in decisions amongst cardiologists.17 Potvin et al. demonstrated in 201 unselected patients for invasive coronary angiography, the use of an FFR threshold of ≤ 0.75 was safe to allow deferral of stenting. However, only 21 % of patients had a recent ACS and the use of FFR was neither blinded nor randomised.40
One of the earliest randomised studies that specifically addressed the utility of FFR-guided decision making in NSTEMI was performed by Leesar et al.41 They reviewed the effect of treatment decisions guided by FFR against stress perfusion scintigraphy. Seventy patients with single-vessel disease and a history of unstable angina were recruited. There was a significant reduction in duration and cost of index hospitalisation in the FFR arm. However, this was not a genuine NSTEMI population as only two-thirds of patients had a diagnosis of AMI, patients were medically stable for 48 hours or more, and the impact of multivessel disease was not assessed.
The FAME study showed that in patients with multivessel disease there was a 30 % reduction in adverse cardiac events (death, MI, target- vessel revascularisation) with an absolute risk reduction of 5 % in the group undergoing FFR-guided PCI compared with those undergoing angiography-guided PCI.42 A secondary analysis in 2011 clarified that the benefit observed in the overall trial population was also seen in the UA/NSTEMI group. Overall, FAME included 328 patients with UA/ NSTEMI of whom 178 were randomised to angiography-guided PCI and 150 to FFR-guided PCI. An absolute reduction in adverse cardiac events of 5.1 % was observed in the FFR-guided group as well as less contrast usage and on average one stent fewer per patient (1.9 ± 1.5 versus 2.9 ± 1.1, P<0.01).36 However, this was merely a subgroup analysis of the larger FAME study with a lack of data on the degree of myocardial injury reported and with patients being stable prior to randomisation. In a separate observational study of 106 patients with NSTEMI, PCI was deferred if the culprit vessel FFR was >0.75. The one-year event rate was 1.9 %, mortality 0.9 %, target-vessel revascularisation and 4.7 % for readmission with a cardiac cause.43
FAMOUS-NSTEMI
The most recent and arguably the largest randomised trial to date examining the role of FFR in patients with NSTEMI was recently published.44 The Fractional Flow Reserve versus Angiographically Guided management to optimise outcomes in Unstable Coronary Syndromes the British Heart Foundation FAMOUS–NSTEMI trial44 was performed across six PCI centres in the United Kingdom. Patients with confirmed Type 1 NSTEMI45 who were planned for an early invasive strategy were randomised to two arms: FFR-guided PCI versus angiography-guided PCI. All patients were to undergo FFR assessment to each vessel containing at least one ≥30 % stenosis, but only in the FFR-guided arm was the result revealed to the operators.
The primary outcome was to assess the between group differences in the proportion of patients allocated to medical management—it was not powered for major adverse cardiac events (MACE). Secondary outcome measures included feasibility, cost and safety assessment, outcome data and quality of life data.
One hundred and seventy-six patients were in the FFR-guided arm and 174 were in the angiography-guided arm. Following coronary angiography and before randomisation, the treating interventional cardiologist was asked to provide a treatment plan based on the coronary angiogram. In the FFR disclosed group this treatment decision was then re-evaluated in light of FFR findings. The median time to angiography from symptom onset was three days but patients were still included in the study if they presented with NSTEMI within 24 hours. Despite a ‘delay’ in angiography this was by no means a low-risk population. An independent ECG core lab demonstrated that 80 % of the population had ischaemic ECG changes. All patients had a positive troponin and 92 % had anginal symptoms at rest. The median GRACE score was 175.
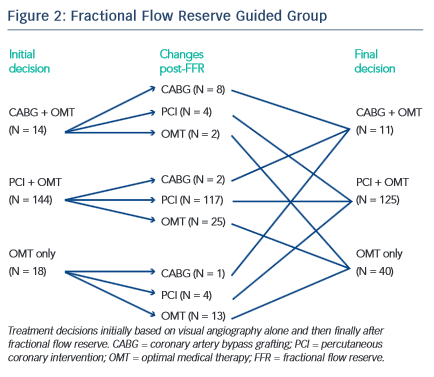
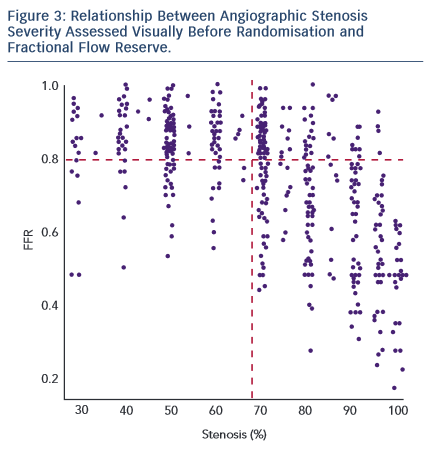
The study demonstrated that there was a significantly higher proportion of patients assigned to medical therapy in the FFR group compared with the angiography group (22.7 % versus 13.2 %, difference 9.5 % [95 % CI: 1.4 %, 17.7 %], p=0.022). An FFR strategy resulted in a change in management decision for 38 (21.6 %) patients (see Figure 2). As was the case with FAME, a major proportion of stenosis that appeared significant visually had been misinterpreted when FFR results were revealed (see Figure 3). This is an important factor that highlights the difficulty in making decisions based purely on the angiogram.
There was no significant difference in MACE between groups at 12-month follow up: FFR-guided 13 (7.4 %) versus angiography-guided 16 (9.2 %) p=0.56. However, when reviewing MACE in patients not related to revascularisation, there was a trend towards a greater number of events, 10 (5.7 %) in the FFR group versus 5 (2.9 %) in the angiography group, p=0.25. Of these 10 patients, four were medically- managed patients on the basis of FFR changing treatment plan due to a value of >0.80. However these events occurred considerably later in follow-up (3–11 months) and therefore may be related to progression of coronary disease rather than issues over the validity of FFR result.
In terms of safety, there were a total of eight coronary dissections of which only two were attributable to the FFR wire and six to percutaneous coronary intervention. This mirrors the 200-patient RIPCORD study that had a total of three coronary dissections related to FFR use.46 Finally, whilst there was a higher upfront cost of FFR, there was no significant overall cost through the index hospitalisation.
Ongoing Clinical Trials of FFR Guided PCI in Patients with ACS
Despite the theoretical considerations outlined above, the weight of evidence suggests that non-culprit FFR can provide useful information regarding functional significance in a high proportion of patients with ACS. On this basis, FFR-guided decision making in patients with STEMI and MVD is now being tested in a series of randomised controlled trials. The Comparison between FFR Guided Revascularisation versus Conventional Strategy in Acute STEMI Patients with MVD (COMPARE ACUTE) study is a randomised controlled trial (RCT) in STEMI patients with MVD in the Netherlands, with estimated enrolment of 885 patients, divided into immediate FFR-guided complete revascularisation versus staged non-culprit PCI (ischaemia-driven) by proven ischaemia or recurrent symptoms. The primary endpoint will be a composite of death, non-fatal MI, CVA or revascularisation at 12 months and this study is estimated to end in 2018.
The Complete versus Culprit-only Revascularisation to Treat Multi- vessel Disease After Primary PCI for STEMI (COMPLETE) study is an RCT-comparing FFR-guided revascularisation within 72 hours of primary PCI versus optimal medical therapy for the endpoint of a composite of cardiovascular death or MI at four years. It began recruiting in 2012 and is due to report its findings in 2018.
The Primary PCI in Patients With ST-elevation Myocardial Infarction and Multi-vessel Disease: Treatment of Culprit Lesion Only or Complete Revascularisation (PRIMULTI) study is an RCT of patients with STEMI and MVD with a comparison of the clinical outcome after complete FFR-guided revascularisation versus treatment of the infarct-related artery only during primary PCI. The primary outcome is all-cause death, MI or revascularisation at 48 months. This study, based in Denmark, has finished recruiting and is due to report its findings later this year.
Conclusion
The use of FFR in patients with acute coronary syndromes remains a controversial area but recent data from the FAMOUS-NSTEMI study have provided a significant advance in the utility of FFR for NSTEMI at a time when its applicability was considered futile due to physiological barriers. We await the findings from ongoing trials to determine the effectiveness of and FFR-guided treatment strategy in STEMI and also a larger outcome-powered study in NSTEMI.
Unless otherwise noted, ™ indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE MEDICAL and the nine-squares symbol are trademarks and service marks of St. Jude Medical, Inc. and its related companies. © 2015 St. Jude Medical, Inc. All Rights Reserved. SJM-OCT-0515-0007a | Item for Global use | Articles courtesy of Dr Jamie Layland